UNL Single IRB Reliance Point of Contact
For questions about the information below, or to inquire about a new reliance request, proposal, sIRB plan, or if you are a representative of an external IRB or institution, please reach out to:
Mikaela Myers
unlreliance@unl.edu
(402)472-8196
UNL investigators often engage in research with project partners at other institutions. In order to document this collaboration for IRB projects reviewed at the Expedited or Full Board levels, IRBs at all involved institutions sign a Reliance Agreement, sometimes referred to as an Institutional Authorization Agreement (IAA). This agreement documents respective authorities, roles, responsibilities, and communication between an institution/organization providing the ethical review and a participating site relying on a Single IRB (sIRB).
Important Notes:
- The IRB Institutional Official, or their designee, is the only individual authorized to enter into single IRB reliance agreements. Investigators are not authorized to enter into agreements on behalf of the institution or the IRB.
- The UNL IRB generally does not enter into reliance agreements for exempt research.
- If it is determined that UNL will be the Reviewing IRB, a New Project Form should be submitted for review. If UNL is the Relying IRB, a Determination Form should be submitted requesting to rely on an external IRB.
- The information contained on this webpage is specific to the UNL IRB. Other NU institutions, including University of Nebraska-Omaha, University of Nebraska Medical Center, and University of Nebraska-Kearney have their own policies specific to single IRB reliance.
- Multi-site or Cooperative research, which involves collaborative efforts from multiple investigators from across institutions, offers a number of benefits including combining diverse expertise and resources leading to innovative outcomes, cross-disciplinary insights, and the potential for broader societal impact. Serving as the Lead PI on multi-site or cooperative research also entails a number of substantive responsibilities associated with supervising research across multiple institutions while meeting relevant regulatory requirements. Please see our note below on the benefits, and associated responsibilities, of serving as the Lead PI on cooperative research.
- Given that the execution of a reliance agreement involves multiple stakeholders (including Reviewing IRB, Relying IRB(s), Reviewing Site Study Teams, and Relying Site Study Teams), each playing crucial roles at specific stages in the process, any delay in action from any of these parties can significantly impede the overall timetable for agreement execution.
- To facilitate the prompt execution of reliance agreements, Lead PIs are encouraged to actively engage in project administration, ensuring that relying site PIs promptly provide their IRBs with the requisite documentation for making a determination to cede review to an external IRB.
- Single IRB reliance is only specific to the IRB review process. All ancillary reviews, including conflict of interest, bio-safety, radiation safety, and other institutional approvals typically are required to be completed per each Institution, as applicable.
Definitions:
- Reliance Agreement: A formal, written document that provides a mechanism for an institution engaged in non-exempt human subjects research to delegate institutional review board (IRB) review to an IRB affiliated with another institution or an independent IRB. Reliance agreement, IRB Authorization Agreement (IAA), cede review, cede, or External IRB are all terms that refer to a situation where research is conducted at two (or more) institutions, and one is designated to serve as the reviewing institution/IRB while the other(s) serves as the relying institution/IRB.
- Single IRB (sIRB): An Institutional Review Board that has been designated as the IRB of record. This IRB is responsible for overseeing all sites participating in a multi-site study.
- Multi-site: Under the NIH Single IRB Review policy, multi-site is defined as two or more sites; hence, a multi-site study is a non-exempt human subjects research study conducted according to a single protocol but at more than one site, and, therefore, carried out by more than one investigator. In other words, the same protocol is being performed by investigators at multiple institutions.
- Cooperative Research: Research involving two or more sites working on different components of a single project and not necessarily using the same research protocol. For example, Institution A might be responsible for data analysis, while Institution B is responsible for recruitment and data collection and Institution C is designated as the coordinating site.
- Relying Institution/Participating Site: A non-UNL site that is engaged in non-exempt research and has ceded IRB oversight to UNL.
The following page is split into three sections:
- Before Reliance. This section discusses when reliance agreements are necessary, the type of reliance agreements that could be used, and considerations when planning your project.
- During Reliance. This section discusses the actual reliance process, including what is expected of investigators both at UNL and other collaborating institutions.
- After Reliance. This section discusses continued investigator responsibilities following the signing of a reliance agreement, including procedures for annual updates, changes to the protocol, and other considerations including reporting of unanticipated problems, serious adverse events, and non-compliance.
Quick Reference
The following chart provides a general overview of the reliance process. More specific guidelines can be found in subsequent sections.
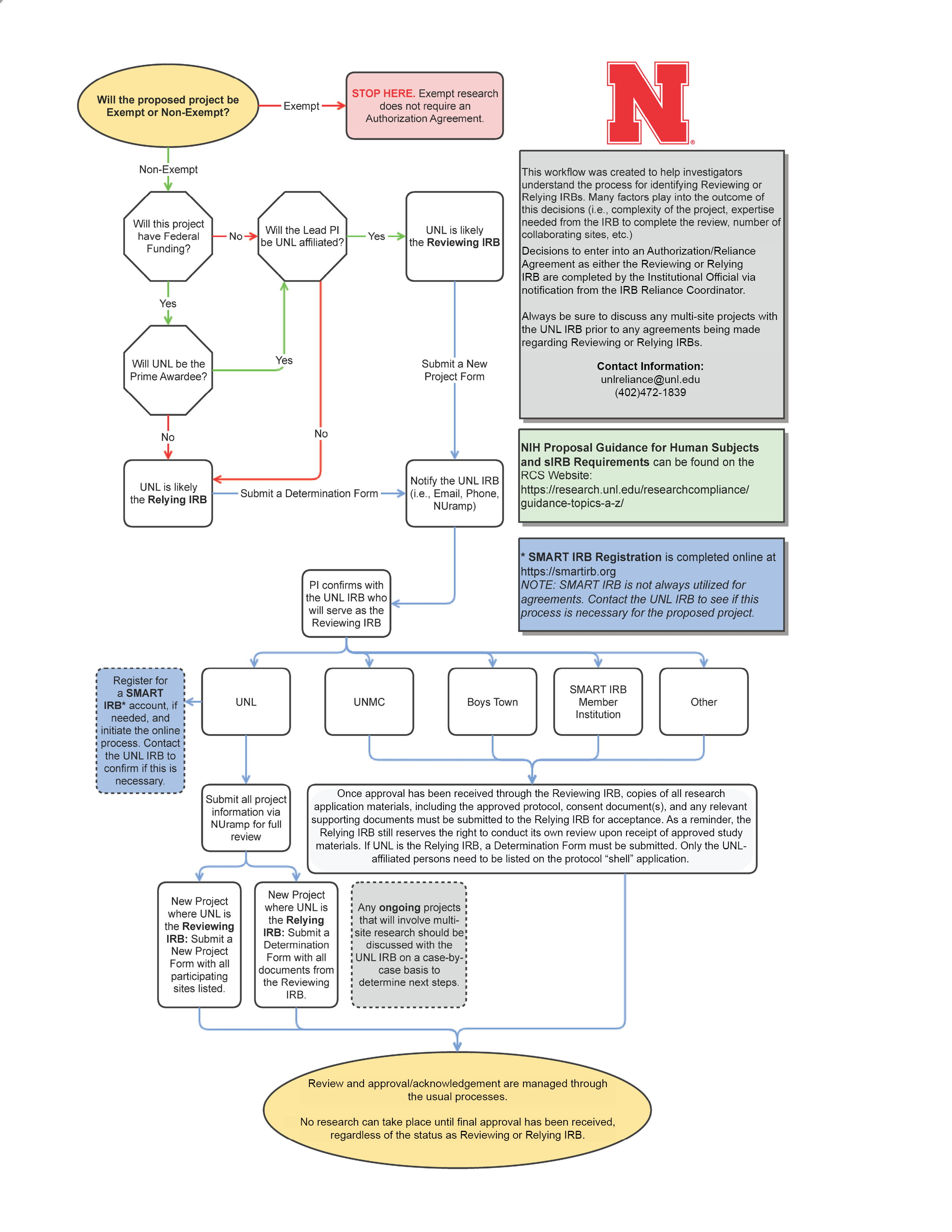
NuRamp Guidance
Submitting a Request for UNL to Rely on Another Institution’s IRB Oversight Guidance Document
Note: These instructions apply only to projects that have been determined to be non-exempt by the Reviewing IRB. If you are applying for IRB oversight on a project that was determined to be exempt by the lead site IRB, you must submit a new IRB application for exempt IRB review.
Before Reliance
When is a Reliance Agreement Needed?
Cooperative research, which involves collaborative efforts from multiple investigators from across institutions, offers a number of benefits. By leveraging the collective expertise, resources, and perspectives of multiple institutions, cooperative research can enhance research outcomes and positively impact diverse populations.
A reliance agreement is required whenever more than one domestic institution is considered engaged in either cooperative or multi-site federally funded research. Institutions may also choose to enter into reliance agreements for non-federally funded non-exempt research at the discretion of the institution. International participating sites are not required to comply with any sIRB requirements. Instead, in these cases, a local ethics committee review is completed to ensure local customs and norms are addressed.
Furthermore, the non-UNL institutions must be engaged in the research for a reliance agreement to be necessary. Please be aware, each institution may apply the “engagement” guidance differently. It is the responsibility of the relying institution to determine if their institution is engaged. Please refer to the following flowchart to determine whether an institution is engaged in research to assist in sIRB planning:
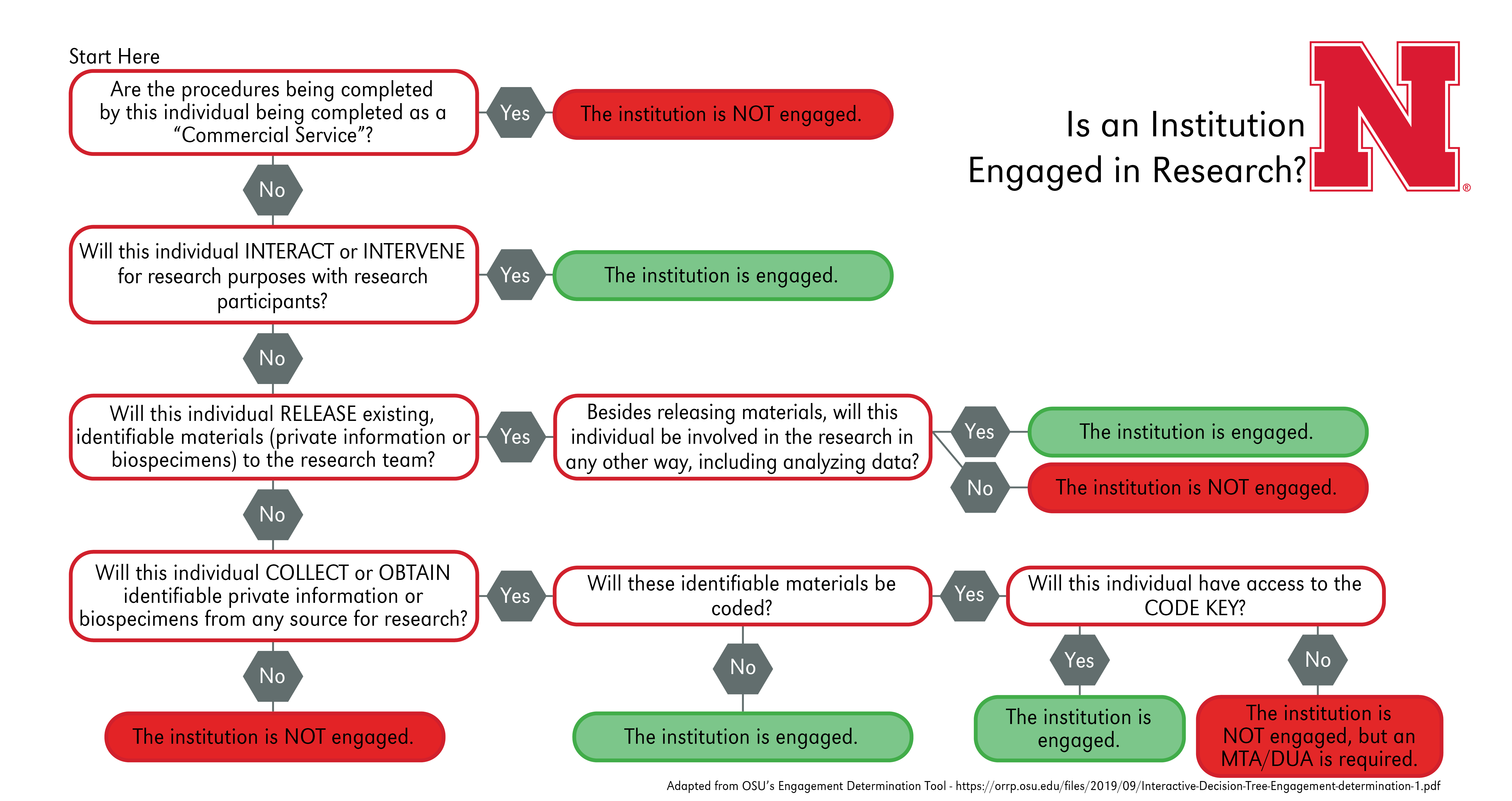
Commercial Service: For an activity to be considered a commercial service, all of the following must be true:
- The external personnel will not receive professional recognition or publication privileges for the services provided;
- The external personnel typically perform the services for non-research purposes; AND
- The external personnel will not administer any intervention being tested or evaluated under the protocol.
Interactions: These occur when investigators communicate or have interpersonal contact with research participants, for example verbally, in writing, or electronically, to obtain information about them for the research.
Interventions: On the other hand, these include both physical procedures by which investigators collect information or biospecimens and manipulate the subjects or the subjects’ environment for the purpose of the research.
If multiple institutions are involved with a research project, but only UNL is considered engaged in the human subjects research activities, no reliance agreements are necessary. Instead, please refer to our Site Permission Documentation A-Z Guidance entry to determine whether an institution is considered a recruitment or performance site for your research and what documentation may be needed. For more information regarding a Materials Transfer Agreement (MTA) or a Data Use Agreement (DUA), please refer to our Secondary Information and Biospecimens: Use in Research and IRB Applicability A-Z Guidance entry. This guidance also specifies who to contact if one of these agreements is necessary.
What Types of Projects Require Agreements?
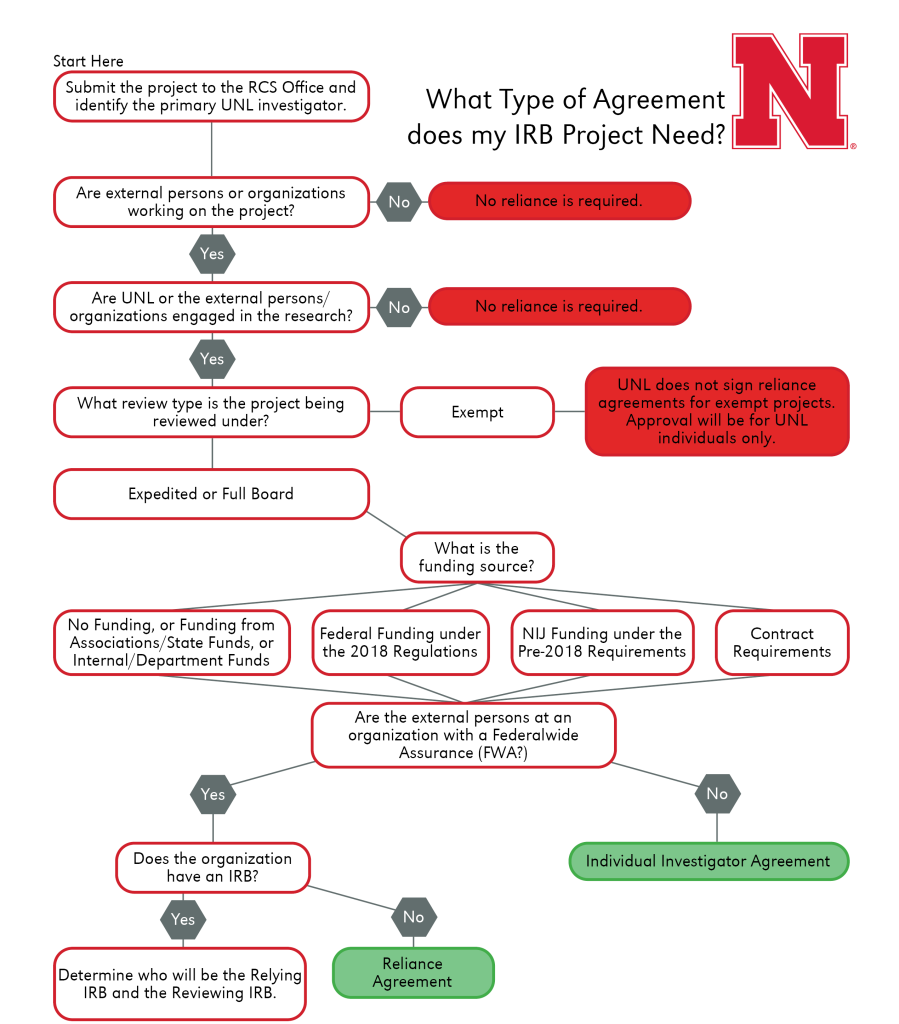
Individual Investigator Agreement
As described in UNL HRPP Policy 3.017, Individual Investigator Agreements, the IIA is an agreement between UNL and an external collaborating independent investigator or a collaborating institutional investigator that does not hold an FWA and does not regularly conduct human subjects research. In these cases, the external collaborator will sign an agreement with UNL agreeing to complete research under UNL’s Federal Wide Assurance and IRB policies. If it is determined that an IIA is necessary, RCIS will work with investigators to prepare and complete the document.
Individual Investigator Agreement Information and Forms
Reliance Agreement
This is the agreement that documents respective authorities, roles, responsibilities, and communication between an institution/organization providing the ethical (IRB) review and a participating site relying on the Single-IRB (sIRB). UNL, per policy, will only complete a Reliance Agreement for non-exempt research (i.e., Expedited or Full Board review).
UNL holds blanket agreements with the University of Nebraska Medical Center as well as Boys Town National Research Hospital. The agreements allow us to meet requirements both under HHS regulations and FDA regulations.
Considerations for Investigators
When developing your project, consider whether there is a need for reliance if at all. The reliance process can be time consuming, especially when coordination is required between multiple institutions. The following are scenarios to demonstrate when reliance would and would not be necessary.
Note: If a project involving multiple institutions is federally funded AND has been reviewed as non-exempt (i.e., Expedited or Full Board), a reliance agreement is required regardless of other project specifics.
Example 1 – Non-Federally Funded Cooperative Research
Researchers from separate institutions are working on a cooperative research project funded with non-federal research start-up funds. In this project, researchers from one institution are completing one-on-one interviews with students about their eating habits. The project is going to be reviewed using the Expedited review process because researchers are not fully disclosing the true purpose of the project (a process known as incomplete disclosure) until after the interview. The interviews will be transcribed and analyzed to look for common trends. Researchers from the second institution are only going to be assisting with analyzing the transcripts.
The second institution would only be considered engaged (and therefore require a reliance requirement) if they are receiving and analyzing identifiable data, as they are not interacting or intervening with participants. However, if they only receive transcripts that are de-identified prior to sharing and are not given access to any identifiable data or identifiable project records, the institution would not be engaged, and a reliance agreement would not be required.
Important Note: It is recommended that the second institution submit a Determination Form or similar form to their respective IRB so that all involved institutions are aware of potential research activities being performed by their affiliated members.
Example 2 – Non-Federally Funded Multi-Site Project
A non-federally funded multi-site project involving dozens of institutions is collecting blood samples from participants before and after a maximal effort exercise regimen and collating data in a central storage location. Each site is completing the same analysis and the results are entered into a spreadsheet. Personally identifiable information (PII) is being collected for a variety of purposes.
It is important to consider whether the sharing of PII is absolutely necessary. Each site could collect PII for their internal use (for example, for compensation, or to complete a health history questionnaire), but if they only share de-identified data outside of the institution, a reliance agreement would not be required. Each site would conduct their own IRB review and approval of the study.
Example 3 – Federally Funded Project
A federally funded multi-site research project with researchers at separate institutions is examining the effects of an anti-bullying campaign in their community and interviewing children about their experiences with bullying. Each institution will de-identify their transcripts and data prior to sharing with other institutions. The project is reviewed using the Expedited review process. Even though de-identified data is being shared with other institutions, this project would still require reliance due to the fact that the overall project is receiving federal funding.
Form Submission
Investigators at all involved sites should notify their respective IRBs of their potential need for reliance as early as possible or based on the institution’s policies and standard operating procedures. The UNL Lead investigator should submit one of two forms, depending on whether they are requesting UNL serve as the reviewing or relying institution. Depending on their institutional policies, some institutions may not require the use of a reliance agreement in certain circumstances. Once all institutions have determined they are willing to enter into a reliance agreement, the IRB points of contact will work together to determine the best timelines for reliance based on project specifics and project needs.
- For projects where UNL has agreed to become the Reviewing IRB, a new project form should be submitted. This form should outline the full scope of activities in which collaborating investigators will be engaged, including recruitment, data collection, data storage, etc. This information is necessary as the Reviewing IRB is assuming responsibility for reviewing the research activities conducted at all collaborating sites. The New Project Form will become the project’s “parent protocol.”
- For projects where UNL is expected to be the Relying IRB, a determination form should be submitted with a request to cede review. This form should provide the scope of activities that UNL-affiliated individuals will be completing as part of the overall project.
Obtaining full IRB approval for a multi-site project can be a lengthier process, as relying sites may need to be provided with the full approved protocol and consent language before they formally agree to serve as a relying institution. Accordingly, the approval of the parent protocol will be followed by an onboarding process, within which collaborating sites agree to rely on the Reviewing IRB for human subjects research. As resources, timetables, and requirements can vary substantially across institutions, PIs should budget for the additional time required in receiving approval for collaborating institutions when developing projected timetables.
During Reliance
Once it is determined that UNL will enter into a reliance agreement as either the Reviewing or Relying IRB, investigators will need to complete additional steps as requested by either the UNL IRB or the Reviewing IRB (when UNL is a Relying site). Please be aware that no research can begin at UNL until an appropriate approval letter is in place from UNL. This includes when UNL is a Relying Site.
Local Context Review: It is important that all applicable laws, regulations, and applicable policies at each institution are considered during IRB review and site onboarding. To obtain this information, institutions may be asked to complete a local context review. Different institutions may have different considerations or requirements. If UNL is a Relying site, UNL’s sIRB reliance coordinator will work with investigators and the Reviewing IRB as needed to provide requested information or supply authorized signatures.
Where the collaborating site has provided local context information relevant to conducting research at a collaborating site, the Reviewing site or Lead PI is responsible for ensuring that those requirements are reflected in the research procedures applicable to that site outlined in the research protocol. The Reviewing IRB will provide researchers with any local context information provided by the relying institution.
Execution of Individual Investigator Agreements: For non-exempt research, when external investigators that are not affiliated with an institution which holds a Federalwide Assurance will be integrated into the study team, individual investigator agreements will need to be executed with each of these individuals. The Reviewing Site PI is responsible for ensuring that each of these investigators provides the Reviewing IRB with the documentation required in HRPP Policy # 3.017 – Individual Investigator Agreements.
Investigator Responsibilities: To ensure a timely review, it is the responsibility of investigators to ensure that all UNL-affiliated project personnel have completed the necessary human subjects training, have completed the Conflict of Interest statement (and have a management plan in place should a conflict be identified), and obtaining any additional ancillary reviews that may be necessary (radiation safety, biosafety, etc.).
During review, investigators are responsible for ensuring that the consent language required by collaborating institutions is adequately integrated into the consent forms utilized for the project. Subsequent revision may be required to ensure that the consent language meets HRPP policy requirements while reflecting the requirements of the collaborating institution.
The Lead PI is responsible for obtaining approval for all site-specific documentation (e.g., consent, advertisements, communication with participants, etc.) and disseminating that documentation to collaborating investigators.
Once the reliance agreement is ready to be executed, the UNL HRPP will ensure that the agreement is appropriately signed (by Institutional Officials at all institutions involved, or their delegate) and will maintain a copy of the finalized agreement. The UNL HRPP or the Lead PI will facilitate communication with Relying IRBs or the Reviewing IRB as required by the executed agreement, standard operating procedures and the agreed upon sIRB communication plan.
After Reliance
Once parent protocol approval has been received, reliance agreements are executed per site, and applicable sites are added to the project and onboarded, the project can commence at each site, as applicable. When UNL is the Reviewing IRB, the following items must be completed as necessary:
Modifications to the Protocol: Where a modification to the research procedures at a collaborating site is required, the Reviewing Site PI is responsible for obtaining approval from the Reviewing IRB before those changes can be implemented. This is completed via a Change Request on the approved project protocol.
Maintenance of Project Personnel: In most cases, the Relying IRB is responsible for verifying, in coordination with the Reviewing IRB point of contact, that site investigators or study team personnel meet the institutional requirements for the relying institution, including education, training, and qualifications to perform the research and safeguard the rights and welfare of research subjects. The Relying IRB is also responsible for disclosing any conflict of interest related to research conducted under a reliance agreement and providing applicable management plans to the Reviewing IRB.
Reporting Protocol Deviations, Violations, Unanticipated Problems, and Adverse Events at Collaborating Sites to the Reviewing IRB: The Reviewing Site or Lead PI is responsible for communicating reportable events to the Reviewing IRB, even where those events occur at collaborating sites. Thus, the PI will need to maintain active lines of communication with their collaborating investigators to ensure that events are reported promptly to the Reviewing IRB. Where additional information is required, the Reviewing PI will need to liaise with the collaborating institution to ensure that information is provided to the Reviewing IRB in a timely manner commensurate with the reporting time requirements and seriousness of the report.
Continuing Review/Annual Update: Annual Update or Continuing Review requirements must be met yearly. These forms are submitted based on the project’s review type and Reviewing IRB institutional requirements.
Final Report/Project or Site Closeout: When the project has been completed, a Final Report must be submitted to properly close out the project.
If UNL is the Relying IRB: Updates to UNL, as a relying institution, should be submitted specific to UNL as the site applicable to the type of documentation submitted upon completion of review by the Reviewing IRB. For example, if a change that applies to UNL is approved, this should be submitted via a change request form to the UNL IRB as a means to keep the Institution updated on the status of the study. The change, however, could be implemented without the UNL approval given that this is an institutional update.
Standing or Master Reliance Agreements

- It is important to note that SMART IRB is not another IRB, but rather an online reliance agreement tracking system and communication tool for IRB administrators.
- PI’s will need to register in order to gain access. This process takes about 2 business days to approve, so advance notice and planning will be important.
- If a partnering institution is a member of SMART IRB, UNL may require the use of this system to manage the agreement process.
- PI’s can see if collaborators are registered members by checking the SMART IRB website.
Additional Guidance and/or External Resources:
- SMART IRB website
- Request investigator access to SMART IRB
- Online Reliance Walkthrough Video
- Searchable SMART IRB Resources
UNL and UNMC, as well as UNL and Boys Town National Research Hospital, have standing master agreements to fluidly cede review to the respective institutions without having to sign study specific agreements. The decision to serve as a Relying or Reviewing IRB is made on a project-by-project basis. All projects must be submitted via the same procedures provided above. (This does not imply that Boys Town and UNMC have a master agreement between their organizations.)
Research can only begin if all other required approvals have been obtained (i.e., radiation safety, biosafety, conflict of interest, etc.). For questions related to multi-site or cooperative research agreements, please contact unlreliance@unl.edu or call (402)472-8196.






