The University of Nebraska-Lincoln’s (UNL) Human Research Protection Program (HRPP) and the projects that are approved through the IRB must follow, Federal regulations, state regulations, institutional policies. The individual HRPP policies and procedures listed below are the most up-to-date versions.
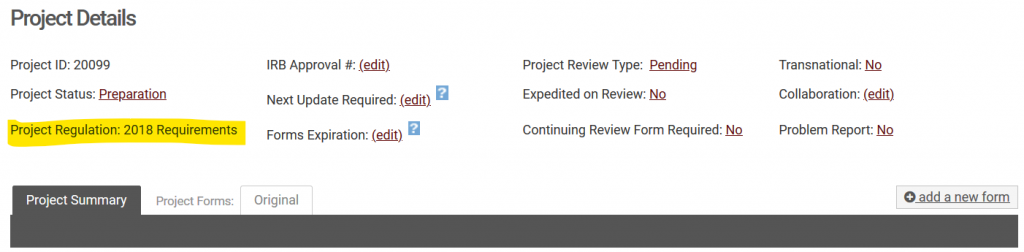
Revisions to the Common Rule (45 CFR 46) went into effect as of January 21, 2019. We refer to these revised policies as those following the “2018 requirements”. If you have an active and ongoing project that was approved prior to January 21, 2019, your project will likely fall under the “Pre-2018” requirements. The one exception to this is for projects funded by the Department of Justice (National Institute of Justice). Because the DOJ is not a signatory of the Revised Common Rule, these projects are reviewed using the Pre-2018 requirements. To find which set of requirements your project is subject to, simply navigate to your project in NuRamp and you will see the “Project Regulation” listed in the project details.
Additional Resources:
- List of common abbreviations found in UNL HRPP policy documents
- Guidance Topics (A-Z) page
Comprehensive Policy Documents (PDF of all policies and procedures):
Organizational Commitment to the HRPP
1.001: The Institution and its Commitment to the HRPP
1.003: Vision, Mission and Values Statement of UNL
1.004: Vision, Mission and Values Statement for the HRPP
1.005: IRB Charter, Appointments and Administrative Structure
1.006: Authority Granted by UNL to the IRB Operating in the HRPP
1.007: Human Research Protection Program Emergency Preparedness Plan
IRB Membership & Standard Operating Procedures
2.001: IRB Membership Requirements and Responsibilities
2.002: IRB Meetings and IRB Member Responsibilities
2.004: Orientation and Initial Training for New IRB Members and HRPP Staff
2.005: Reserved
2.006: Continuing Education Requirements for IRB Members and HRPP Staff
2.007: Evaluation of IRB Members
2.008: IRB Member Confidentiality
2.009: Full Board IRB Reviewer Assignment
2.010: Written Reviews by IRB Members and Development of the IRB Review Letter
2.011: IRB Quorum and Voting Requirements
2.013: HRPP Policy Review and Approval
2.015: Ongoing Research Transition to 2018 Requirements
Initial IRB Review of Protocols
3.001: Investigational Activities Requiring IRB Review and Approval
3.002: Ethical Principles Governing Research Under the Jurisdiction of the IRB
3.003: Initial Application Submission
3.004: Criteria for IRB Approval of Research
3.005: IRB Initial Review Categories
3.006: Scientific and Scholarly Merit Review of Proposals
3.007: Reserved
3.008: Qualification and Responsibilities of Research Personnel
3.009: Required Training in the Protection of Human Participants
3.011: Certificate of Confidentiality
3.012: IRB Approval of Multi-Site or Cooperative Research
3.013: Research Records Retention and Security
3.014: PI Disagreements with IRB Decisions
3.015: Research Participant Payment and Incentives
3.016: Recruitment of Participants
3.017: Individual Investigator Agreements
3.018: Human Participant Research Involving Ionizing Radiation
3.019: International Council for Harmonization – Good Clinical Practice (ICH-GCP) E6R2 Compliance
Exempt & Expedited review
4.003: Exempt Research – Limited Review
Vulnerable Populations and Special Classes of Participants
5.001: Additional Protections for Vulnerable Populations
5.002: Research Involving Pregnant Women, Human Fetuses and Neonates
5.003: Research Involving Prisoners
5.004: Research Involving Children
5.006: Research Involving Extra Credit Compensation
Section 6
Reserved
Random Compliance Review
General Requirements and Guidelines
8.001: Students as Researchers
8.002: Epidemiological Research Guidelines
8.003: Exercise Protocol Guidelines
8.004: Research Conducted in Foreign Countries
8.005: Community-Based Participatory Research
8.006: Enhancing Understanding of Participants, Prospective Participants, and the Community
8.008: Pregnancy Testing of Females of Childbearing Potential
8.009: Use of Deception in Research
8.010: Banking Human Biological Material for Future Research
8.011: Use of Human Biological Material in Research
8.012: Participant Registries, Subject Pools, and Data Repositories
8.013: Registering Clinical Trials (ClinicalTrials.gov)
Informed Consent
9.001: Required Elements for Informed Consent Documents
9.002: Development of the Informed Consent Document
9.004: Re-consent/Assent Research Participants
9.005: Absence of Valid Consent: Re-Consent and Use of Data
9.006: Waiver or Alteration of Consent and Consent Documentation
9.007: Use of the Short Form Consent Document
Protected Health Information & Research
10.001: Use of Protected Health Information in Research and Registries
Continuing Review
11.001: Continuing Review and Annual Update
11.002: IRB Disapproval, Study Hold, Suspension, and Termination
11.003: Minimization of Risk and Data Safety Monitoring
Amendments to Approved Protocols
Reportable New Information
13.002: Study Related Complaints
Compliance
14.003: Audits by Outside Agencies
Funding Requirements
15.001: Human Research Compliance with Department of Education Regulations
15.002: Human Research Compliance with Environmental Protection Agency Regulations
15.003 Reserved: The Department of Justice (DOJ) is not a signatory of the Revised Common Rule. Please see the Pre-2018 policy #15.003 for HRPP Requirements.
15.004: Human Research Compliance with Department of Defense Regulations
15.005: Human Research Compliance with Department of Energy Regulations
Conflict of Interest
16.001: Conflict of Interest (COI) Identification and Management
16.002: Reserved






