Electrical and Computer Engineering
Tiffany Lee, July 26, 2021
Researchers develop simple, inexpensive method for guarding carbon fiber
For the past 50 years, manufacturers have considered carbon fiber a dream material: Though individual fibers are thinner than a strand of human hair, they can be twisted together and fused with a matrix material to form a lightweight composite that is stronger than steel, twice as stiff and a good conductor of heat. And, unlike metals, the material doesn’t crack over time. It’s been used in a wide range of applications, including air and spacecraft, cars, buildings, medical devices and sports equipment.
But carbon fiber has a major drawback, said Husker engineer Yongfeng Lu, an expert in carbon materials. Under extreme temperatures — encountered routinely in the aerospace industry, for example — carbon fiber oxidizes, meaning it reacts with oxygen in the air and burns, just as wood does when combined with enough heat and oxygen. Oxidation quickly diminishes the dream-like qualities of carbon fiber, particularly its strength.
“One weakness of carbon fibers is that they are burned easily if you have high enough temperatures and oxygen present,” said Lu, Lott Distinguished University Professor of electrical and computer engineering. “If we could make them non-flammable, so that they don’t burn when exposed to fire, that would be exciting.”
In a recent paper published in PNAS, Lu’s team describes a major step toward that goal. He and colleagues from the University of Nebraska–Lincoln and the Institute of Condensed Matter Chemistry of Bordeaux in France developed a low-cost, scalable method for guarding carbon fiber against oxidation. The approach represents a significant improvement over other antioxidation processes that are laborious, slow and expensive.
“We are trying to add surface layers that can separate carbon fibers from oxygen so that even under high temperatures, they won’t be burned,” Lu said. “Carbon fibers can be used in many ways — woven into textiles and in parts of buildings, airplanes, electronics equipment — but if they’re flammable, that poses a new risk to the system and limits those applications a lot.”
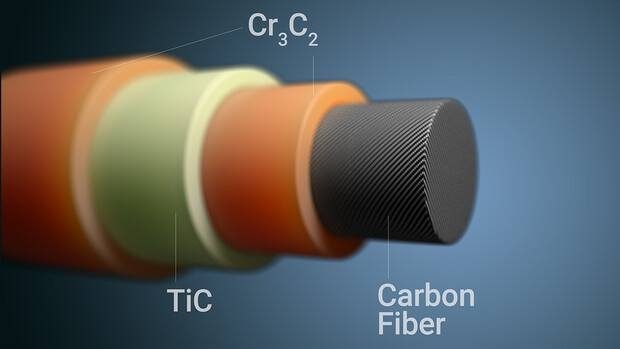
To eliminate flammability, Lu’s team has devised a simple, single-step process that starts with melting a salt that is very similar chemically to table salt. After the salt crystals become a liquid, the researchers add titanium and chromium powders, which are known to withstand high temperatures. Carbon fibers are then added to the mix.
After a spontaneous reaction, the process yields a three-layer coating — made of chromium carbide and titanium carbide — that serves as a barrier against oxidation. The coating is multilayered because titanium and chromium each have different behaviors and reaction rates within the molten salt, leading to three distinct layers of end product. This triple coating confers extra protection when compared to a single layer.
When the researchers evaluated the coated carbon fibers against extreme temperatures — about 2,200 degrees Fahrenheit — and extreme environmental conditions they simulated with an oxyacetylene flame, they found that the carbon material maintained its structure. Lu said the next step is identifying just how fireproof the coated fibers are compared to their unprotected counterparts, and how long they can retain their most valuable properties under extreme conditions.
Lu’s team isn’t the first to explore methods of protecting carbon fibers against oxidation, but if successful under further testing, the approach would be the first with large-scale viability. Previous approaches, such as chemical vapor deposition, involve expensive equipment, multiple steps and chemical reactions that are difficult to control. The molten salt approach circumvents these pitfalls by using basic, cheap materials that undergo a spontaneous process at a relatively low temperature of about 1,800 degrees Fahrenheit.
The process is also fast and clean, poising it for widespread industrial use.
“We’ve found a recipe that can form three layers in a single state,” Lu said. “With one single dip, we can get three layers of coating.”
Other members of the team include Loic Constantin, graduate student at Nebraska’s Laser Assisted Nano Engineering Lab; Lisha Fan, postdoctoral researcher in LANE; Bai Cui, associate professor of mechanical and materials engineering; and Jean-François Silvain, adjunct professor of electrical and computer engineering and research director of the Institute of Condensed Matter Chemistry of Bordeaux.
The National Science Foundation and Nebraska Research Initiative supported the work.