Mechanical and Materials Engineering
Scott Schrage, February 27, 2020
To the point: Mini-needles may help smart bandages better heal wounds
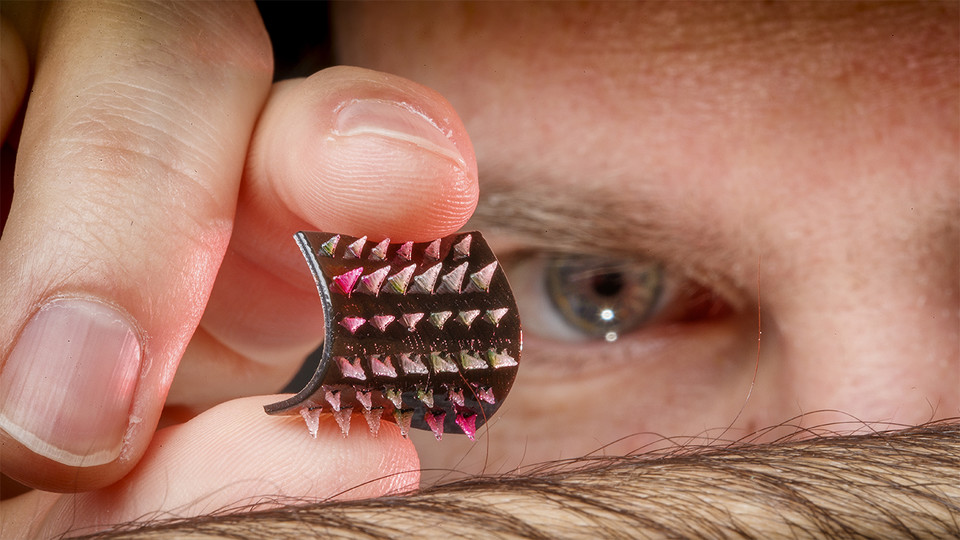
A new smart bandage design could eventually heal chronic wounds by getting under a patient’s skin — where an array of tiny needles can deliver therapeutic drugs directly to the still-living but damaged tissue.
Developed at the University of Connecticut and University of Nebraska–Lincoln, the 3D-printed prototype consists of a polymer bandage equipped with millimeter-scale needles, reservoirs and pumps that can be wirelessly controlled via smartphone or other Bluetooth-enabled device.
By plunging past the dead surface-level tissue that can persist for months in people with Type 2 diabetes, those needles could administer drugs to help close wounds, reduce infection, stimulate cell growth and potentially restore blood flow. Limiting the duration of such wounds, the researchers said, could also help dethrone them as the leading non-traumatic cause of amputations.
“This is an important step in engineering advanced bandages that can facilitate the healing of hard-to-treat wounds,” said Ali Tamayol, the lead engineer who conceived the design at Nebraska before moving to Connecticut.
The design piggybacks on a series of prior smart bandage prototypes co-developed by Tamayol. Though the new design shares many features with its predecessors — including control over the dose and delivery schedule of individual drugs — all of those predecessors administer drugs only to the surface of the skin.
“If we want to actually claim ‘smart’ control, then that means everything can be controlled precisely by the user, rather than relying on diffusion — so you control the flow of the drug into the body,” said Ruiguo Yang, assistant professor of mechanical and materials engineering at Nebraska.
A procession of pitfalls lurking beneath the skin’s surface may explain why prior prototypes have failed to facilitate healing in clinical trials, the researchers said. Many of the problems begin with blood vessels breaking open near a wound. Without those vessels ferrying essential nutrients and oxygen to the wound, the surrounding skin tissue begins dying. Inflammation, bacterial infection and other issues soon follow.
Those factors and failings suggested that healing chronic wounds could be as much a matter of “where” as it is “what” and “when,” inspiring the team’s new approach. To test it, the researchers recreated a chronic wound by growing a layer of crusty, dying skin tissue atop a still-viable one.
After loading a standard gel-covered bandage and the needle-equipped counterpart with the same biological agent, then covering separate wounds with each bandage for three hours, the team analyzed the viable tissue for the presence of the agent. Whereas the miniature needles delivered 70% of the agent down to the viable tissue, the gel-covered bandage managed to transport just 1%.
Though encouraging, the experiment told the researchers little about whether their feat might translate to faster, more complete healing of chronic wounds. So they turned to a group of diabetic mice with skin wounds similar to those that afflict an estimated 4.5 million Americans, and millions more worldwide, each year.
After about three weeks of being treated with tissue-regenerating growth factors, wounds covered by the needle-equipped prototype had closed by an average of 95%, compared with 55% when covered by a topical bandage. Skin tissue at the wound’s center appeared to regenerate about twice as thickly, with the surface boasting more hair and less scarring. And that central tissue showed higher levels of a protein associated with blood vessel formation.
The team acknowledged that much work remains: surmounting the engineering challenges of integrating sensors, electrodes and wires; considering other bandage and needle materials that might better accommodate large-scale production; and, most importantly, putting its design to the test in human clinical trials.
But Yang said the early results are a legitimate source of optimism for addressing a problem that resists both healing and easy answers.
“We’re very excited about this,” he said. “Deep down, we know that the things we’re doing and building, to be able to deploy them in a medical setting to treat patients, is maybe far down the road. However, if you look back (over time), all the small, small, small improvements led to what we have today.
“I always remind my students that they need to read a lot of history, at least the history of their field, so that they know that they have a place, albeit small, in that history.”
The team detailed its prototype and findings in the journal Advanced Functional Materials. Tamayol and Yang authored the study with Nebraska’s Seth Harris, Craig Kreikemeier-Bower, Fariba Aghabaglou, Azadeh Mostafavi, Ian Ghanavati and Jordan Rosenbohm; Nebraska alumni Hossein Derakhshandeh, Alec McCarthy, Chris Wiseman and Zack Bonick; Harvard Medical School’s Dennis Orgill and Pooria Mostafalu; and Seyed Masoud Moosavi Basri of Shahid Beheshti University.
The researchers received support from the National Institutes of Health under grants GM126831, AR073822 and P20GM113126, along with the National Science Foundation and the Nebraska Tobacco Settlement.