Scott Schrage, October 20, 2023
Husker chemists, engineers craft adjustable arrays of microscopic lenses
DESIGN COULD FIND USE IN SOFT ROBOTICS, LIQUID OPTICS
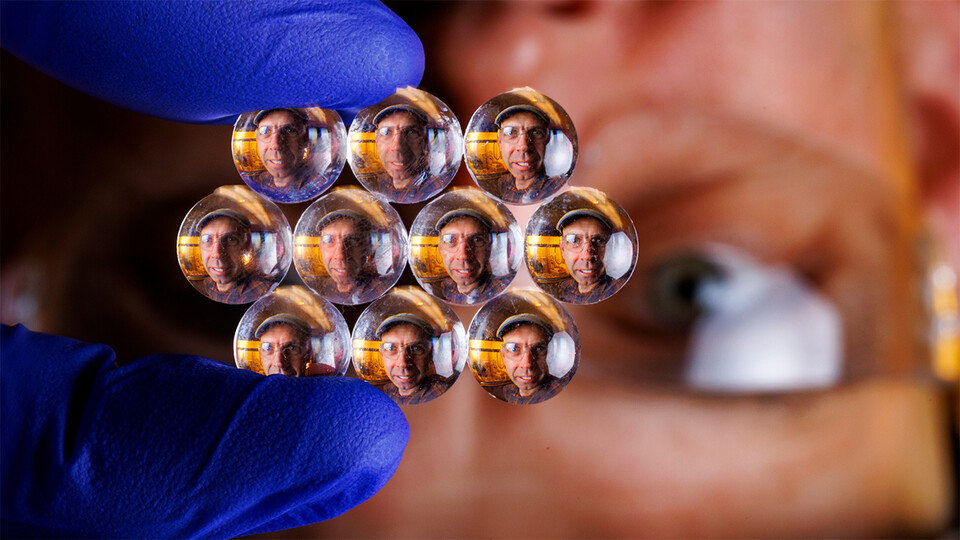
They number in the thousands, light striking the phalanx of lenses arrayed on a face in geometric pattern, the beams refracting through transparent mounds no wider than a hair.
A fly’s eye boasts roughly 4,000 microscopic lenses, a honeybee’s up to twice that many. These lenses, though, belong not to a compound eye but to polydimethylsiloxane — a flexible polymer long ranking as a favored playground of Nebraska’s Stephen Morin and his band of fellow chemists.
With the aid of engineers Ruiguo Yang and Grayson Minnick, Morin’s team can now arrange and affix tiny gelatinous lenses to an elastic material that accommodates an even grander achievement. By carving the equivalent of aqueducts into the material, then running temperature-altering or water-gathering fluids through those channels, the researchers can also expand or contract the lenses in mere seconds — modifying their magnification, focal length and other optical properties in the process.
Whereas insects and crustaceans evolved their multifaceted eyes to draw in panoramas of ancient environments, Morin’s team is envisioning the future: projecting signals onto sensors embedded in soft robotic skins, for instance, via on-demand control.
“The artificial micro-lenses we have today are relatively static,” said Morin, associate professor of chemistry at the University of Nebraska–Lincoln. “They have a fixed focal length, a fixed size. They are made from materials that give you the lensing property you want, but they don’t really have any dynamic characteristics.”
To add that missing dynamism, Morin and his colleagues turned to hydrogels, the class of water-infused polymers that lend soft contact lenses their pliability. In the past, the team had physically adhered hydrogel islands to silicone materials, a deceptively difficult feat in its own right. But enough agitation, or the introduction of enough water, would inevitably detach the islands from their silicone base.
“The problem is that putting those together in a way that they function synergistically is not well-established,” Morin said. “There wasn’t really anything out there that was putting these two materials together in a robust, long-term platform.”
Overcoming the challenge, Morin knew, would mean supplementing the physical connection with a chemical one. Doctoral student John Kapitan and the team began by priming the transparent silicone with a patterned plasma treatment, coating it with strategic molecular groups and a lithium-based compound, depositing the hydrogel islands, then later applying just the right wavelengths of ultraviolet light. That light initiates the release of highly reactive free radicals that hopscotch across various molecular groups, essentially propagating chains that protrude both up from the silicone itself and across the emerging structure, stabilizing it.
“When it’s all said and done,” Morin said, “you have a somewhat monolithic structure.
“Now, in addition to that physical part, there’s this chemical element. And that was really the secret sauce.”
Morin and his colleagues would “aggressively” put the monolith through its paces. They threw water on it. They stretched the silicone, twisted it. They slapped on pieces of tape and peeled them off, trying to take the lenses with them. They even gave it an ultrasonic bath, peppering it with frequencies often used to clean jewelry, electronics and other grime-attracting products. The microscopic lenses hung tough through it all.
“When we were done, we were pretty satisfied that they were stuck on there pretty well,” Morin said.
Another series of experiments, led by doctoral student Brennan Watts, would soon test and demonstrate the lenses in action. In one, the team shone light on a Nebraska N, projecting it onto an array of hydrogel lenses and, beyond them, a microscope positioned to view the resulting images. When the researchers ran cold water through the material supporting those lenses, the Nebraska N appeared sharp, in focus. Just seconds after cranking that water up to 178 degrees Fahrenheit, the lenses shrank and, on cue, the N blurred out of focus.
To its surprise, the team would later learn that the shift in focus stemmed not from the changing size or curvature of the lenses, but instead mostly from an alteration to their so-called refractive index. Light travels at different speeds when passing through different media — air, water, the human eye — and those changes in speed correspond to the light refracting, or bending, at different angles. As the hydrogel heated up and the lenses contracted, they actually expelled some of their water — upping their density, modifying their refractive index and, ultimately, blurring the image of the N.
While Morin said that on-the-fly adaptability bodes well for the design’s use in micro-projection systems, the chemist is also intrigued by its potential applications in biology. Because hydrogel generally mimics the gelatinous network residing between the cells of complex organisms, researchers often favor it when attempting to culture cells or tissues outside a biological environment.
A device designed by Yang, associate professor of mechanical and materials engineering, has granted Morin’s lab precise control over not just the size, patterning and composition of the hydrogel lenses it deposits, but the orientation and tension of the silicone they reside on, too. That precision, combined with the team’s ability to reversibly manipulate the lenses themselves, might expand the culturing options available to those working in biomaterials and biomedical engineering, Morin said.
“It would seem reasonable that these types of dynamic changes in size and stiffness and things of that nature would have a profound effect on the biology of anything contained in them,” he said. “We’re not there yet, but we certainly have interest in those problems.”
For Morin, who’s spent years experimenting with silicones and other polymers, the practical considerations of adaptable materials are informing, and informed by, the philosophical. There was, he said, a sensible reason for attaching the hydrogel lenses to silicone: Its elasticity relieves some of the stress imposed by the swelling and shrinking of the lenses, helping it maintain a longer-term grip than other, more brittle materials might.
But the chemist is also keen on reconsidering the physical and functional rigidity of what gets made — of viewing materials and structures through a new lens, give or take a few thousand.
“There’s some confusion, I think, as to why we want materials that adapt,” he said. “And I think that’s built into the way that we’ve designed and manufactured materials … going all the way back to whenever we first started making things, I suppose.
“I always make the argument that it would be great if, 100 years from now, the materials we made were able to adapt as we grow and change, as opposed to just being designed to stay the same the whole way through. Of course, this work is just a microcosm of that. But that’s the idea. That’s what adaptive materials could give us.”
The team, which also included Nebraska’s Nengjian Huang and Mark Rose, reported its findings in the journal Advanced Functional Materials. The researchers received support from the Army Research Office and the National Science Foundation.