Nebraska Center for Materials and Nanoscience
Scott Schrage, April 5, 2021
Magnetic breakthrough could lower power use, up speed of digital memory
“It was a very painful process to get to this point,” said Christian Binek, before explaining how, over the hairpin-turned, speed bump-lined course of 15 years, toil gave way to triumph.
Like so many research teams around the globe, a University of Nebraska–Lincoln group led by Binek, Peter Dowben and Alexei Gruverman sought what Binek called a “holy grail”: a quantum material whose magnetic states could be altered by electric means alone, and above room temperature.
Now, finally, with the help of a chemical impurity and a less-is-more epiphany, the team has crafted a nanoscopic material and microscopic configuration capable of just that. As Binek sees it, the material — chromium oxide with a dash of the impurity, boron — could help herald the emergence of digital memory and processors that consume far less power, while potentially running even faster, than their modern-day counterparts.
Chromium oxide belongs to an exclusive club of materials that exhibit a phenomenon called antiferromagnetism. The atoms in any magnetic material act like tiny bar magnets, each with a north and south pole. Most materials that are traditionally considered magnetic are actually ferromagnetic, with the magnetic poles of every atom pointing in the same direction to produce an easily measurable magnetic field. Antiferromagnets, by contrast, feature alternating columns of atoms whose poles point in opposite directions — up-down-up-down, for instance — and effectively cancel each other out, yielding virtually no magnetic field.
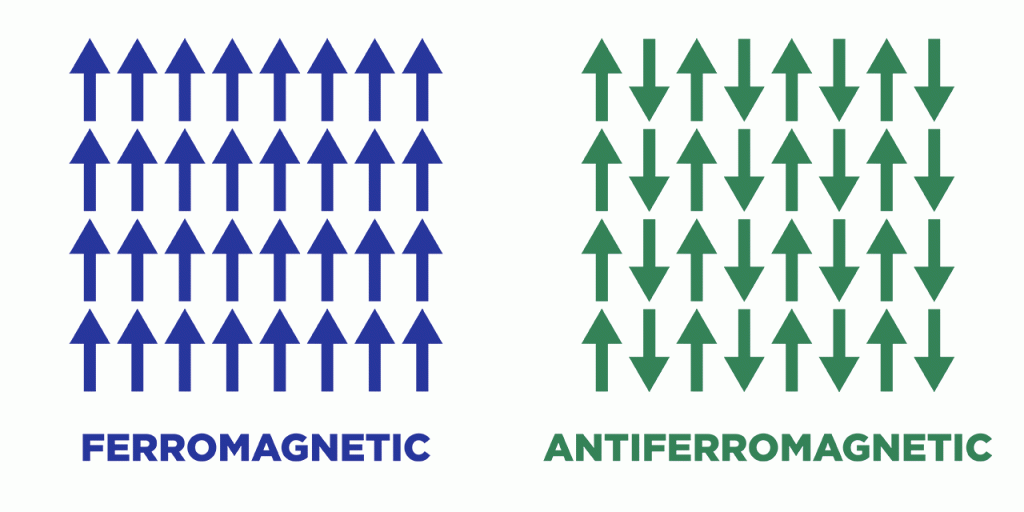
The internal magnetic states of both ferromagnets and antiferromagnets can be switched to encode the 1s and 0s of binary data. But antiferromagnets especially appeal to electronic engineers, for multiple reasons. Their lack of external magnetism nullifies the chance of digital components interfering with each other. Many, including chromium oxide, naturally insulate against the stifling heat generated inside digital devices. Most promisingly, their internal magnetism can be switched not just by external magnetic fields but by electric ones, the latter of which demand substantially less power and also eschew the pesky interference issue.
Just one problem: Though chromium oxide is a champion at withstanding heat and switching magnetic states, it actually loses its antiferromagnetism — and its willingness to be manipulated by electric fields — above 93 degrees Fahrenheit.
“Let’s say you want to think about integrating a memory device that you bring anywhere near a CPU,” said Binek, Charles Bessey Professor of physics at Nebraska. “That gets hot. You cannot afford for your device to lose all function at (93 degrees).”
To solve the not-so-small problem, Binek and his colleagues first pursued an approach that involved creating a nanoscopically thin layer of an antiferromagnetic material — chromium oxide — and topping it with a ferromagnetic one. Their logic was sound: use an electric field to dictate the state of the antiferromagnet, which would then modify the magnetism of the ferromagnet above it, which would then produce a magnetic signature that could be read as a 1 or 0. After adding boron to the chromium oxide, the approach raised the temperature threshold, a major feat in its own right. Even then, though, the team still had to apply an energy-hungry magnetic field. And even that wasn’t enough.
“The force that kept the ferromagnet coupled to the antiferromagnet was too weak,” said Binek, who directs the Nebraska Center for Materials and Nanoscience. “So we were forced to try something else. And that ended up working to our advantage.”
That something else was to get rid of the overlying ferromagnet entirely. The team discovered that an antiferromagnet alone produced just enough of a surface-level magnetic signature — generated by its atomic magnets reorienting by 90 degrees, rather than the usual 180 — to be read as a bit of data.
It worked at higher temperatures, up to 260 degrees Fahrenheit. It worked by applying only voltage, without the assistance of an external magnetic field. Not only that: The same weakness of the force that frustrated the team’s previous approach was also making it easier to switch the internal magnetic orientations of the antiferromagnet. A theoretical physicist working with the team has estimated that the switching could occur in as little as 100 picoseconds, roughly 10 times faster than the nanosecond required of a typical ferromagnetic material.
“The time it takes to reverse the magnetization of a ferromagnet is a limiting factor in a device,” Binek said. “A nanosecond sounds fast, but for modern devices, it’s too slow. The fact that we no longer need a ferromagnet means that this problem is now also eliminated.”
How the projected 100-picosecond speed translates to actual devices is uncertain, Binek said, but an encouraging early sign. And whether or not it actually stores and processes data faster than ferromagnets, its major advantage in energy-efficiency could make it a workhorse in devices that are typically untethered from a power source.
“Take the Internet of Things,” Binek said, referring to WiFi-enabled devices and appliances. “If you want objects to have memory, you want to do everything with low energy, and you want … to have the possibility to switch it off without the system forgetting the state it is in. And that is also fulfilled in our case.
“So you can make a list of checkboxes,” he said, “and this checks them all off.”
The team recently reported its findings in the journal Nature Communications. Binek, Dowben and Gruverman authored the study with Nebraska’s Ather Mahmood, Will Echtenkamp, Mike Street, Jun-Lei Wang, Shi Cao, Takashi Komesu, Pratyush Buragohain and Haidong Lu, along with Arun Parthasarathy of New York University and Shaloo Rakheja from the University of Illinois at Urbana-Champaign.
The researchers received support in part from the Army Research Office; the National Science Foundation, which funds Nebraska’s Materials Research Science and Engineering Center; and the Nebraska Research Initiative. Samples were fabricated at the Nebraska Center for Materials and Nanoscience and the Nebraska Nanoscale Facility.
Nebraska Center for Materials and Nanoscience Nebraska Nanoscale Facility Physics