Scott Schrage, January 6, 2023
Eating viruses can power growth, reproduction of microorganism
Study first to investigate, demonstrate effects of ‘virovory’
Over a single day, in the placid waters of a pond, a million virus particles might enter a single-celled organism known for the minuscule hairs, or cilia, that propel it through those waters.
Over the last three years, the University of Nebraska–Lincoln’s John DeLong has been diving into a potential tide-turning secret: Those virus particles are not just a source of infection, they’re also nutrition.
In a turnabout worthy of Pac-Man, DeLong and his colleagues have found that a species of Halteria — microscopic ciliates that populate freshwater worldwide — can eat huge numbers of infectious chloroviruses that share their aquatic habitat. For the first time, the team’s lab experiments have also shown that a virus-only diet, which the team calls “virovory,” is enough to fuel the physiological growth and even population growth of an organism.
Chloroviruses, a career-defining discovery by Nebraska’s James Van Etten, are known to infect microscopic green algae. Eventually, the invading chloroviruses burst their single-celled hosts like balloons, spilling carbon and other life-sustaining elements into the open water. That carbon, which might have gone to predators of the tiny creatures, instead gets vacuumed up by other microorganisms — a grim recycling program in miniature and, seemingly, in perpetuity.
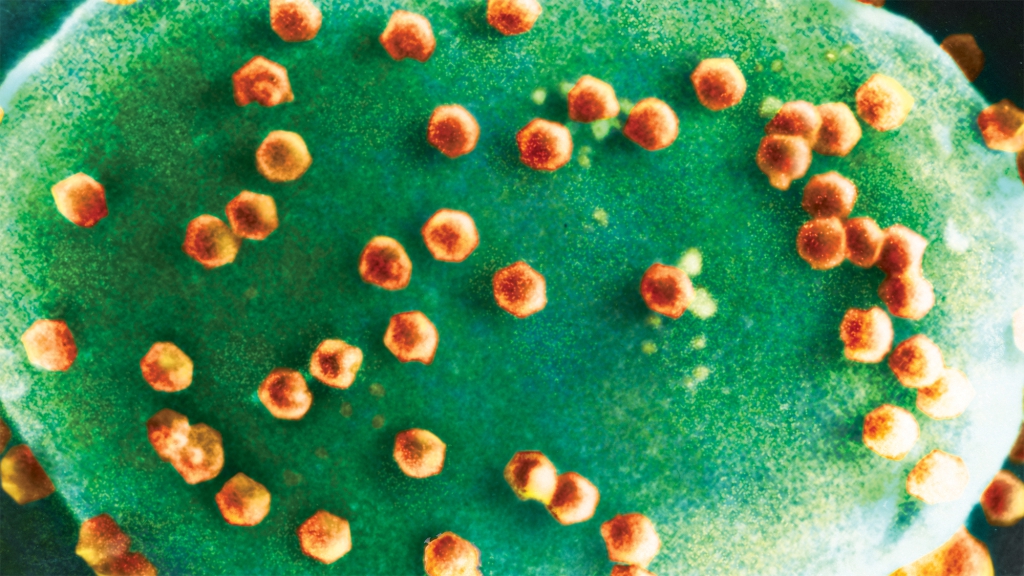
“That’s really just keeping carbon down in this sort of microbial soup layer, keeping grazers from taking energy up the food chain,” said DeLong, associate professor of biological sciences at Nebraska.
But if ciliates are having those same viruses for dinner, then virovory could be counterbalancing the carbon recycling that the viruses are known to perpetuate. It’s possible, DeLong said, that virovory is aiding and abetting carbon’s escape from the dregs of the food chain, granting it an upward mobility that viruses otherwise suppress.
“If you multiply a crude estimate of how many viruses there are, how many ciliates there are and how much water there is, it comes out to this massive amount of energy movement (up the food chain),” said DeLong, who estimated that ciliates in a small pond might eat 10 trillion viruses a day. “If this is happening at the scale that we think it could be, it should completely change our view on global carbon cycling.”
‘Nobody noticed it’
DeLong was already familiar with the ways that chloroviruses can entangle themselves in a food web. In 2016, the ecologist partnered with Van Etten and virologist David Dunigan to show that chloroviruses gain access to algae, which are normally encased in a genus of ciliates called Paramecia, only when tiny crustaceans eat the Paramecia and excrete the newly exposed algae.
That finding put DeLong in “a different headspace” when it came to thinking about and studying viruses. Given the sheer abundance of viruses and microorganisms in the water, he figured it was inevitable that — even setting aside infection — the former would sometimes wind up inside the latter.
“It seemed obvious that everything’s got to be getting viruses in their mouths all the time,” he said. “It seemed like it had to be happening, because there’s just so much of it in the water.”
So DeLong dove into the research literature, intent on surfacing with any studies on aquatic organisms eating viruses and, ideally, what happened when they did. He emerged with precious little. One study, from the 1980s, had reported that single-celled protists were capable of consuming viruses, but delved no further. A handful of papers from Switzerland later showed that protists seemed to be removing viruses from wastewater.
“And that was it,” DeLong said.
There was nothing about the potential consequences to the microorganisms themselves, let alone the food webs or ecosystems they belonged to. That surprised DeLong, who knew that viruses were built not only on carbon but other elemental cornerstones of life, too. They were, at least hypothetically, anything but junk food.
“They’re made up of really good stuff: nucleic acids, a lot of nitrogen and phosphorous,” he said. “Everything should want to eat them.
“So many things will eat anything they can get ahold of. Surely something would have learned how to eat these really good raw materials.”
As an ecologist who spends much of his time using math to describe predator-prey dynamics, DeLong wasn’t entirely sure how to go about investigating his hypothesis. Ultimately, he decided to keep it simple. First, he’d need some volunteers. He drove out to a nearby pond and collected samples of the water. Back at his lab, he corralled all of the microorganisms he could manage, regardless of the species, into drops of the water. Finally, he added generous portions of chlorovirus.
After 24 hours, DeLong would search the drops for a sign that any species seemed to be enjoying the company of the chlorovirus — that even one species was treating the virus less like a threat than a snack. In Halteria, he found it.
“At first, it was just a suggestion that there were more of them,” DeLong said of the ciliates. “But then they were big enough that I could actually grab some with a pipette tip, put them in a clean drop, and be able to count them.”
The number of chloroviruses was plummeting by as much as 100-fold in just two days. The population of Halteria, with nothing to eat but the virus, was growing an average of about 15 times larger over that same timespan. Halteria deprived of the chlorovirus, meanwhile, wasn’t growing at all.
To confirm that the Halteria was actually consuming the virus, the team tagged some of the chlorovirus DNA with a fluorescent green dye before introducing the virus to the ciliates. Sure enough, the ciliate equivalent of a stomach, its vacuole, was soon glowing green.
It was unmistakable: The ciliates were eating the virus. And that virus was sustaining them.
“I was calling up my co-authors: ‘They grew! We did it!’” DeLong said of the findings, now detailed in the journal Proceedings of the National Academy of Sciences. “I’m thrilled to be able to see something so fundamental for the first time.”
DeLong wasn’t done. The mathematical side of him wondered whether this particular predator-prey dynamic, as strange as it seemed, might share commonalities with the more pedestrian pairings he was accustomed to studying.
He started by charting the decline of the chlorovirus against the growth of the Halteria. That relationship, DeLong found, generally fits with those ecologists have observed among other microscopic hunters and their hunted. The Halteria also converted about 17% of the consumed chlorovirus mass into new mass of its own, right in line with percentages seen when Paramecia eat bacteria and millimeter-long crustaceans eat algae. Even the rate at which ciliates preyed on the virus, and the roughly 10,000-fold disparity in their sizes, match up with other aquatic case studies.
“I was motivated to determine whether or not this was weird, or whether it fit,” DeLong said. “This is not weird. It’s just that nobody noticed it.”
DeLong and his colleagues have since identified other ciliates that, like Halteria, can thrive by dining on viruses alone. The more they uncover, the more likely it seems that virovory could be occurring in the wild. It’s a prospect that fills the ecologist’s head with questions: How might it shape the structure of food webs? The evolution and diversity of species within them? Their resilience in the face of extinctions?
Again, though, he’s opted to keep it simple. As soon as Nebraska’s winter relents, DeLong will head back to the pond.
“Now,” he said, “we have to go find out if this is true in nature.”





