Scott Schrage, September 21, 2020
Team IDs compound, gene essential to fungal infiltration of rice plant
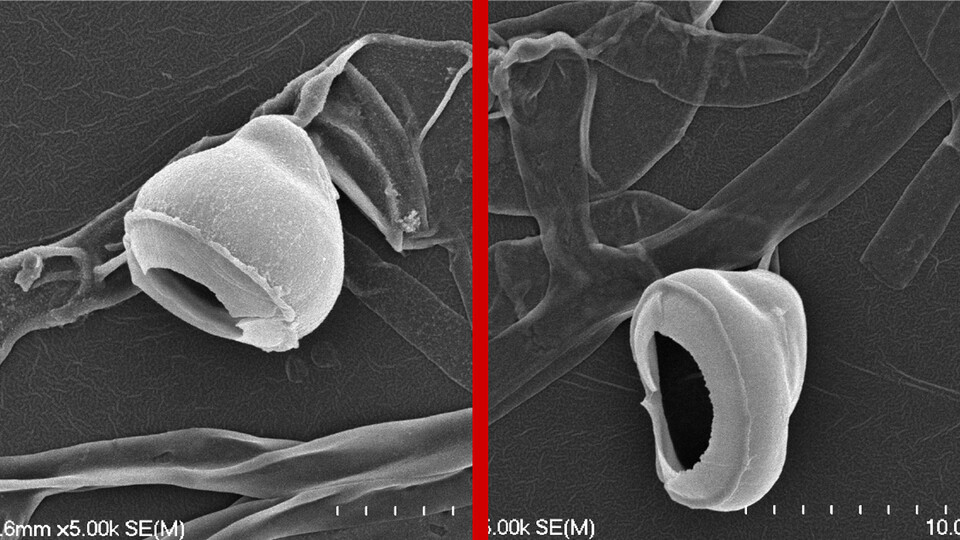
After paragliding in on a breeze, a spore released by the fungus Magnaporthe oryzae settles onto the leaf of an unlucky rice plant.
Over the next 24 hours, the spore goes to work infiltrating its landing pad with a toolkit worthy of Ocean’s 11. It germinates, sprouting a thin tube whose tip morphs into a dome-shaped cell called an appressorium. The appressorium then grows a peg that proceeds to punch through the leaf’s protective film with enough force to puncture Kevlar, granting it access to the tissue inside.

Wilson
“It’s a real brute-force mechanism,” said Richard Wilson, associate professor of plant pathology at the University of Nebraska–Lincoln. “It’s really quite remarkable.”
It’s also quite disastrous for the plant, allowing the so-called rice blast fungus to spoil up to 30% of the world’s potential rice yields in a given year.
But new insights from Wilson and his colleagues could eventually inform novel approaches to combating the parasitic fungus. The team found that a glue-like sealant produced by the appressorium — mucilage — is essential to its infiltration. What’s more, the researchers have pinpointed both an organic compound needed to keep the mucilage flowing and a gene responsible for the compound itself.
That compound is spermine. Many fungal species don’t produce it, so when Wilson’s team realized that M. oryzae does, the researchers took note and started to investigate. They eventually managed to track down the gene, SPS1, that directs the fungus to synthesize the compound.
Without knowing spermine’s exact purpose in the fungus, Wilson suspected that deleting the gene might induce a form of illness, limit spore growth or at least restrict the development of their appressoria in some way. Instead, his team discovered that the purpose of the gene and the resulting spermine is much more specific. The spores of the spermine-deprived M. oryzae continued to sprout their appressoria, and those appressoria even continued to grow their leaf-puncturing pegs.
Despite retaining all of its equipment, though, the SPS1-lacking fungus failed to penetrate and infect the leaf. On closer inspection and after multiple experiments, the team traced the failure to a lack of mucilage. Without enough of the viscous, sugar-rich substance, a fungal spore is unable to establish a leak-proof seal around its appressorium. And without a proper seal, a compound known as glycerol can likely seep out, Wilson said, sapping the appressorium of the extreme pressure buildup that drives its peg down through the cuticle of a leaf.

Curious about whether spermine might have a role in other fungi, the researchers searched for the SPS1 gene in other species whose genomes have been catalogued. They found it in multiple others, most of whose spores can also sprout appressoria. Though Wilson’s team has yet to test those other species, he expects that deleting their SPS1 genes could induce a similar response. One of those species can infect the roots of wheat; another attacks the roots of soybean, causing sudden death syndrome.
“Nobody knew what the spermine was doing previously,” Wilson said. “Now it seems to be in other fungi that make these appressoria and not in many fungi that do not. And that just adds some extra weight to our conclusions that the spermine is required.”
Though Wilson said it’s too early to tell how the team’s findings might be applied or how effective those applications might be, he did venture a couple of possibilities.
“One way would be to target, with inhibitors, the spermine part of that (biochemical) pathway,” he said. “If those were applied at the right time, that could prevent the appressoria from sticking down and breaching the leaf.”
An experiment designed to verify that spermine alone is to blame for mucilage production could point to another, more surprising deterrent. After deleting the SPS1 gene from the fungus, the researchers grew the SPS1-deficient strain on plates of growth media containing moderate levels of spermine. When they did, the fungus responded by uptaking the external spermine as its spores formed. Those spores formed appressoria that produced normal levels of mucilage, allowing them to puncture the surface as they otherwise would.
Beyond a certain threshold, though, the external spermine had a very different effect.
“What we found that was quite interesting and counterintuitive is that if we instead added a lot of spermine directly to spores, it prevented the appressoria from forming,” Wilson said. “Spermine is needed inside the appressorium to make mucilage, but if you apply a lot of it outside, it prevents appressorium formation all together. So there may be a (detrimental) level of spermine, which is pretty common and probably a cheap compound just to spray.
“I can’t claim that it would be a silver bullet, but it could be part of a toolkit for addressing these issues.”
The researchers reported their findings in the journal Nature Microbiology. Wilson authored the study with Raquel Rocha, a recent doctoral graduate of plant pathology, undergraduate student Ngo Pham, and Christian Elowsky, assistant professor of practice of agronomy and horticulture.
The team received support from the National Science Foundation.